Abstract
Germline mutations of DEAD-box helicase 41 (DDX41) have recently been implicated in adult-onset myeloid neoplasms, including myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). However, with their prevalence being largely based on the studies from Caucasian populations, the prevalence of DDX41 pathogenic variants in other ethnicities and their risks for myeloid neoplasms have not been fully investigated. Moreover, the clinicopathological features of DDX41-mutated myeloid neoplasms and their genetic profiles have not been elucidated either.
To address these issues, we investigated germline DDX41 variants among a large cohort of Japanese patients (n=1,609) with sporadic myeloid neoplasms including MDS (n=1,102), myelodysplastic/myeloproliferative neoplasms (n=14), myeloproliferative neoplasms (MPN) (n=31), secondary AML derived from these myeloid neoplasms (sAML) (n=47), and de novo AML (n=410), as well as Japanese healthy controls (n=17,186) using targeted deep sequencing, whole exome sequencing, and/or whole genome sequencing, though which pathogenic germline DDX41 variants and their clinical, pathological, and genetic features were investigated. Risk of detected germline variant for myeloid neoplasms was evaluated by comparing their frequencies between patients and healthy individuals. The germline origin of candidate risk alleles was confirmed using buccal mucosa samples.
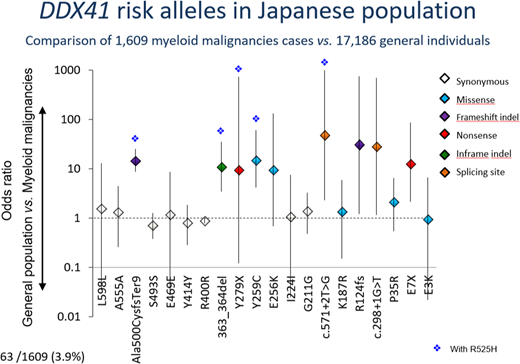
We identified 4 germline variants that were significantly enriched in the patient cohort (n=58, 3.6 %) compared to healthy controls (n=42, 0.2%) (OR=15; 95%CI: 10.2-22.8), including two truncating variants, p.A500fs (OR=14; 95%CI: 8.7-24.4), p.E7X (OR=12.6; 95%CI: 2.1-86), and two missense alleles, p.Y259C (OR=15; 95%CI: 4.1-60), and p.S363del (OR=11; 95%CI: 3.4-35). Four additional truncating and splice-site variants, Y279X (n=1), R124fs (n=1), c.571+2T>G (n=2), and c.298+1G>T (n=1), were also considered as risk alleles, although a small number of each variant precluded an accurate estimation of enrichment in the patient cohort. Overall, as many as 63 (3.9%) patients harbored one of these variants, where each variant allele was invariably heterozygous. Of note, none of these 8 risk variants have been reported in the Caucasian population.
To further evaluate the pathogenic role of these DDX41 germline variants, clinical features and somatic genetic events were investigated. The median age at diagnosis did not significantly differ between patients with and without DDX41 variants (60 and 56 years old in variant carrier and in non-carrier, respectively), suggesting that DDX41-mediated myeloid leukemogenesis shows an age-dependence similar to that in other sporadic cases. Compared with DDX41-unmutated cases, DDX41-mutated cases showed a higher male predominance (52/11 and 987/559, respectively; OR=2.7; 95%CI: 1.4-5.1) and were more likely to have an initial diagnosis of MDS rather than de novo AML (55/8 and 1,094/402, respectively; OR=2.5; 95%CI: 1.2-5.4).
Patients with germline DDX41 mutations had a median of 1 (0-5) somatic mutations. Somatic DDX41 mutations were found in 43 (2.6%) cases, a majority of which harbored a germline DDX41 risk variants (32/63 (52%) and 11/1,549 (0.7%), respectively; OR=143; 95%CI: 67-311). Other targets of somatic mutations in risk allele-positive cases included ASXL1 (n=9), DNMT3A (n=6), TP53 (n=5), and JAK2 (n=4), for which no significant correlation was observed between risk allele-positive and negative cases. Abnormal cytogenetics and copy number abnormalities were detected in 30 (48%) of the patients with DDX41 risk alleles, none of which were significantly in the risk allele-positive cases.
In conclusion, we identified DDX41 germline risk variants among the Japanese population. Germline DDX41 variants were seen in a substantial fraction of Japanese patients with sporadic myeloid neoplasms. Found in the general Japanese population at very low frequencies, these risk alleles account for the largest germline risk for myeloid neoplasms. Somatic DDX41 mutations were common with a prominent mutational hotspot, almost exclusive found in patients with DDX41 risk alleles. Given the high prevalence of DDX41 germline variants and the late onset of associated MDS and sAML, their detection may help better manage patients and carriers who carried DDX41 risk alleles, even when no family history is known.
Ishiyama:Alexion Pharmaceuticals, Inc.: Honoraria. Chiba:Bristol Myers Squibb, Astellas Pharma, Kyowa Hakko Kirin: Research Funding. Asou:Chugai Pharmaceutical Co., Ltd.: Research Funding; Astellas Pharma Inc.: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Asahi Kasei Pharma Co., Ltd.: Research Funding; Eisai Co., Ltd.: Research Funding; SRL Inc.: Consultancy; Yakult Honsha Co., Ltd.: Speakers Bureau; Kyowa Hakko Kirin Co., Ltd.: Speakers Bureau. Naoe:Otsuka Pharmaceutical Co., Ltd.: Research Funding; Toyama Chemical Co., Ltd.: Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; Pfizer Japan Inc.: Research Funding; Astellas Pharma Inc.: Research Funding; Fujifilm Corporation: Patents & Royalties, Research Funding. Usuki:Otsuka Pharmaceutical Co., Ltd.: Research Funding; GlaxoSmithKline K.K.: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Astellas Pharma Inc.: Research Funding; Sanofi K.K.: Research Funding; Shire Japan: Research Funding; SymBio Pharmaceuticals Limited.: Research Funding; Celgene Corporation: Research Funding, Speakers Bureau; Daiichi Sankyo: Research Funding; Sumitomo Dainippon Pharma: Research Funding, Speakers Bureau; Boehringer-Ingelheim Japan: Research Funding; Pfizer Japan: Research Funding, Speakers Bureau; Janssen Pharmaceutical K.K: Research Funding; Novartis: Speakers Bureau; Ono Pharmaceutical: Speakers Bureau; Takeda Pharmaceutical: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau; Nippon Shinyaku: Speakers Bureau; Mochida Pharmaceutical: Speakers Bureau; MSD K.K.: Speakers Bureau. Miyawaki:Novartis Pharma KK: Consultancy; Otsuka Pharmaceutical Co., Ltd.: Consultancy; Astellas Pharma Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal